
Check4®-hCG(hl) for Healthcare Professionals
Check4®-hCG(hl) is a CE-Marked low-sensitivity rapid self-test for detection of human Chorionic Gonadotropin (hCG) level above 1000 mIU/mL in urine to determine the outcome of first-trimester termination of pregnancy (ToP) or miscarriage
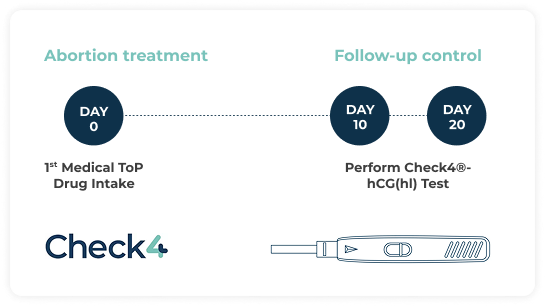
Check4®-hCG(hl) delivers results in under 10 minutes and can be prescribed 10 – 20 days after miscarriage or medical ToP, providing results sooner than classical pregnancy tests.
Check4®-hCG(hl) provides 100% diagnostic sensitivity and 98% diagnostic specificity. The test adheres to the highest quality standards and is trusted by leading healthcare providers in the UK, Europe and Australia.
Designed with simplicity and accuracy in mind, Check4®-hCG(hl) features intuitive design and easy-to-follow instructions, providing reliable and easy-to-interpret results.
Product information
Check4®-hCG (hl) is a rapid qualitative low sensitivity urine pregnancy test (also known as LSUP, LSPT, or LSUPT), authorised for self-use at home.
The test is designed to detect an hCG level of 1000 mIU/mL or more in urine sample, providing clear positive/negative results in 5 – 10 mins.
Evaluation reports show that Check4®-hCG (hl) can detect hCG above 1000mIU/mL in over 99% of cases.
Each pack contains:
- One (1) instruction for use.
- One (1) Check4®-hCG (hl) test packaged in a hermetically sealed aluminium pouch containing one desiccant sachet.
The test must be prescribed by a healthcare professional and is usually performed between 10 to 20 days after:
- First trimester miscarriage
- The first drug intake in Medical ToP
Reasons to Choose Check4®-hCG(hl)
- The first low sensitivity urine hCG self-test to receive CE-mark, developed together with and for clinicians.
- 99% Accuracy when testing on or after the day prescribed by a healthcare professional.
- Trusted by leading and national healthcare providers in the UK, Europe and Australia.
- Made in France by ISO13485 and ISO9001 certified EU manufacturer.
- Enables remote follow-up for medical abortion, with high satisfaction reported by both patients and healthcare providers.
- Extensively validated, CE-marked and registered with global regulators, including the UK Medicines and Healthcare products Regulatory Agency (MHRA) and Australian Therapeutic Goods Administration (TGA).
Trust-Worthy and Accredited
 CE-Marked for at home self-testing
CE-Marked for at home self-testing
 ISO 13485:2016 accredited manufacturer compliant with the internationally recognised standard for QMS in the design and manufacture of medical devices.
ISO 13485:2016 accredited manufacturer compliant with the internationally recognised standard for QMS in the design and manufacture of medical devices.
 Entered in the Australian Register of Therapeutic Goods (ARTG)
Entered in the Australian Register of Therapeutic Goods (ARTG)
 Registered with the UK MHRA
Registered with the UK MHRA
 Manufactured in France within the EU
Manufactured in France within the EU
 CE-Marked in accordance with In-Vitro Diagnostics Directive (IVDD) regulatory framework
CE-Marked in accordance with In-Vitro Diagnostics Directive (IVDD) regulatory framework
What testing method allows patients to quickly and easily confirm whether a termination of pregnancy worked?
| Check4®-hCG(hl) | Standard Pregnancy Test | Laboratory Blood hCG Assay | |
|---|---|---|---|
| Can the test be performed at home by a patient? |
|
|
|
| Can the test be performed 10 days after ToP procedure? |
|
|
|
| Are results available within 10 minutes? |
|
|
|
| Does the test allow for early intervention in case of unsuccessful ToP? |
|
|
|
| Does the test remove the need for an in-person appointment at a clinic? |
|
|
|
Clinical Evidence
The WHO recommends self-management of medical abortion in whole or in part as one of abortion care options at gestational ages <12 weeks. Self-assessment of abortion outcome/success using low-sensitivity pregnancy test (or multi-level pregnancy test) is as effective as assessment by a trained health worker (High-certainty evidence).1
hCG and ß-hCG levels in urine have a similar or slightly higher concentration than those detected in serum2. Urine tests with detection limits of 1000 mIU hCG/mL or higher has been shown to be negative 2 weeks after complete induced abortion.3
Check4®-hCG(hl) has diagnostic sensitivity of 100%, diagnostic specificity of 98% and global correlation of 99% to CE-marked reference method4. The clinical performance of Check4®-hCG(hl) has been extensively validated in several prospective observational studies, demonstrating sensitivity of 100%. 5 6
Since 2014, healthcare providers have been prescribing low-sensitivity pregnancy tests to confirm ToP, supported by multiple retrospective, prospective, randomized, and observational studies demonstrating the feasibility and high acceptance rates for ‘self-assessment’.
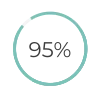 agreement between clinicians and patients on interpreting low-sensitivity pregnancy test results (UK study)5
agreement between clinicians and patients on interpreting low-sensitivity pregnancy test results (UK study)5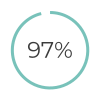 of patients using low-sensitivity pregnancy test correctly comprehend their pregnancy status (US study)7
of patients using low-sensitivity pregnancy test correctly comprehend their pregnancy status (US study)7Check4®-hCG(hl) is a Class IIb in-vitro diagnostic (IVD) medical device, authorised for ‘self-testing’ (which means it can be used by anyone without previous experience of testing and provide reliable results).
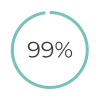 of patients find low-sensitivity pregnancy tests very easy/ easy to use8
of patients find low-sensitivity pregnancy tests very easy/ easy to use8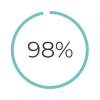 of patients have low-sensitivity pregnancy test with/ without checklist as their preferred follow-up method8
of patients have low-sensitivity pregnancy test with/ without checklist as their preferred follow-up method8Further Information
Learn more about Check4® and our company values.
Discover how healthcare professionals can purchase any of our Check4® tests.
Have an enquiry? Get in touch with a friendly member of our team.
Resources for Healthcare Professionals
References:
- WHO abortion care guidelines. https://www.who.int/publications/i/item/9789240039483
- Stenman UH et al. The classification, functions and clinical use of different isoforms of HCG. Human Reproduction Update. 2006 Nov-Dec;12(6):769-784.
- van der Lugt B et al. The disappearance of human chorionic gonadotropin from plasma and urine following induced abortion. Disappearance of HCG after induced abortion. Acta Obstet Gynecol Scand. 1985;64(7):547-52.
- Data on file
- Michie L, Cameron ST. Simplified follow-up after early medical abortion: 12-month experience of a telephone call and self-performed low-sensitivity urine pregnancy test. Contraception. 2014;89(5):440-445. doi:10.1016/j.contraception.2014.01.010
- Whitehouse KC, Shochet T, Lohr PA. Efficacy of a low-sensitivity urine pregnancy test for identifying ongoing pregnancy after medication abortion at 64 to 70 days of gestation. Contraception. 2022;110:21-26. doi:10.1016/j.contraception.2022.02.005
- Fok WK, Lerma K, Shaw KA, et al. Comparison of two home pregnancy tests for self-confirmation of medication abortion status: A randomized trial. Contraception. 2021;104(3):296-300. doi:10.1016/j.contraception.2021.05.003
- Constant D, Harries J, Daskilewicz K, et al. Is self-assessment of medical abortion using a low-sensitivity pregnancy test combined with a checklist and phone text messages feasible in South African primary healthcare settings? A randomized trial. PLoS One. 2017;12(6):e0179600. doi:10.1371/journal.pone.0179600
*Disclaimers
The images are used for illustration purposes, the appearance of the test and results may differ.
