


Check4® Malaria
Check4® Malaria (35084AG) is a quick, accurate and easy-to-use test for the detection of malaria in whole blood, plasma or serum samples.
Please note that this product is not CE-marked.
Description
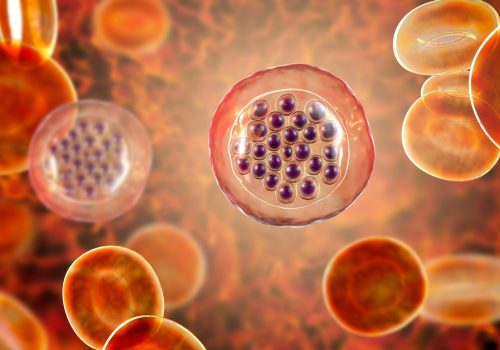
Malaria is one of the most common infectious illnesses in the world. Plasmodium falciparum, accounting for nearly all malaria mortality, kills an estimated 1 to 2 million persons yearly. Recently, a protein called histidine-rich protein (HRP-II) has demonstrated to be a marker of choice for the malaria diagnosis. HRP-II is a protein synthesized by Plasmodium falciparum parasites after erythrocytes infection and during their intracellular growth (6, 7). Check4® Malaria is based on the immunological detection of the histidine rich protein II (HRP-II). The method employs a unique combination of monoclonal-dye conjugate and polyclonal solid phase antibodies to selectively identify HRP-II in whole blood, plasma or serum samples with a high degree of sensitivity.
The box contains the material necessary to perform 10 or 20 tests:
- 1 P.F.-CHECK-1 aluminium pouch 10 20 containing: – 1 P.F. device – 1 disposable capillary pipette (10μL)
- Lysing reagent in a dropper bottle containing buffer, detergent and sodium azide (NaN3 < 0.1%)
- 1 instruction leaflet
All our Check4® range of products are:
- Made and Evaluated in France
- Easy-to-use and easy to read results
- Accurate and trust worthy
- Quick with results in a matter of minutes
Please note that this product is not CE-marked.
Product Specification
| Specification Type | Specification Value |
|---|---|
| Product Range | Professional Test |
| Product Code | 35084AG |
| Product Name | Check4® Malaria |
| Product Analyte | Various |
| Product Size | 20 x Tests |
| Product Storage | 2°C to 30°C |
Check4® Malaria test is based on the immunological detection of the histidine rich protein II (HRP-II). The method employs a unique combination of monoclonal-dye conjugate and polyclonal solid phase antibodies to selectively identify HRP-II in whole blood, plasma or serum samples with a high degree of sensitivity. As the test sample flows through the absorbent device, the labelled antibody-dye conjugate binds to the HRP-II forming an antibody-antigen complex. This complex binds to the anti- HRP-II antibody in the positive reaction zone (T) and produces a pink-rose colour band when HRP-II is present in the sample. In the absence of HRP-II, there is no line in the positive reaction zone (T). The reaction mixture continues flowing through the absorbent device past the reactive zone and control zone. Unbound conjugate binds to the reagents in the control zone (C), producing a pink-rose colour band, demonstrating that the reagents are functioning correctly.
Malaria is one of the most common infectious illnesses in the world. Plasmodium falciparum, accounting for nearly all malaria mortality, kills an estimated 1 to 2 million persons yearly (1). About 40% of the world’s population is at risk for acquiring malaria, resulting in 300 to 500 millions cases yearly, and around 80% of these occur in subsaharan Africa, where malaria kills 1 out of 20 children in rural Africa before they reach 5 years of age.
Check4® Malaria test is designed for whole blood, plasma or serum samples. The specimen should be collected under the standard laboratory conditions and should contain an anticoagulant (EDTA, Heparin…) in case of venous blood. Whole blood samples should be tested immediately (< 4 hours). If the test is to be run within 48 hours after collection, the specimen should be stored in the refrigerator (+2°C to +8°C) with anticoagulant.
1.This test is designed for in vitro diagnostic use and professional use only.
2.Read carefully instruction leaflet before using this test.
3.Handle all specimens as if they contained infectious agents. When the assay procedure is completed, dispose of specimens carefully after autoclaving them for at least one hour. Alternatively, they can be treated with 0.5% to 1% solution of sodium hypochlorite for one hour before disposal.
4.Wear protective clothing such as laboratory coats and disposable gloves while assaying samples.
5. Do not eat, drink or smoke in the area where specimens and kit reagents are handled.
6. Avoid any contact between hands and eyes or nose during specimen collection and testing.
7. Do not use beyond the expiry date which appears on the package label.
8.Do not use a test from a damaged protective wrapper.
9.When the test is performed with whole blood, fresh samples should be used (< 4 hours).
If there is no distinct colour band visible in the test and control area, the test is inconclusive. In this case, it is recommended to repeat the test.
The sensitivity of Check4® Malaria test is 89.5% and the specificity is 93% when compared to the blood smear test which is the gold standard for the Plasmodium falciparum detection. These results show that Check4® Malaria test rapid test is performing extremely well and that it could be used as a very reliable tool for Plasmodium falciparum antigen detection.
For more information on drug abuse and addiction you can visit the below:
- https://www.nhs.uk/conditions/malaria/
- https://www.gov.uk/government/collections/malaria-guidance-data-and-analysis
